Odd battery discharge?
I recently finshed my battery voltage logger. The battery under test is a 12V, 7AH Lead-Acid battery. I've attached a graph to show the anomally. The Y axis is the battery voltage in mV(minus 8V to better show the anomally). The X axis is in seconds. This is a fairly old battery, so that is why all this happened in 1 hour. I believe I was drawing about 1.5A's at the beginning.
Anyway, please not the normal discharge slope. The voltage tends to drop faster and faster as it discharges, which I believe is normal, but note the last minute or so as the voltage rises all of a sudden, then starts back down. I wish I could have logged more, but I programmed the propeller to only record 1 hour worth of discharge.
Any ideas what this last part means?
Anyway, please not the normal discharge slope. The voltage tends to drop faster and faster as it discharges, which I believe is normal, but note the last minute or so as the voltage rises all of a sudden, then starts back down. I wish I could have logged more, but I programmed the propeller to only record 1 hour worth of discharge.
Any ideas what this last part means?


Comments
I suspect the load caused the glitch.
Bean.
▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Uhhhm, that was on fire when I got here...
www.iElectronicDesigns.com
·
Anymore done on your solar panels, Bean?
-Phil
Post Edited (Phil Pilgrim (PhiPi)) : 6/24/2008 7:30:35 PM GMT
Yeah, I spent a good bit of time a year or so back, trying to figure out why no measurements of voltage vs. current worked on a light bulb. I then found that out that the resistance changes, which makes sense considering how HOT that filament is, and that resistance does typically go up with temp(semiconductors have the opposite behavior).
I considered that exact thing about the blip at the time I pulled in the data to excel. That was the first thing that came to mind, but then again, I'm pretty sure my connections were solid. I soldered wires to the bulb and made sure everything was stout.
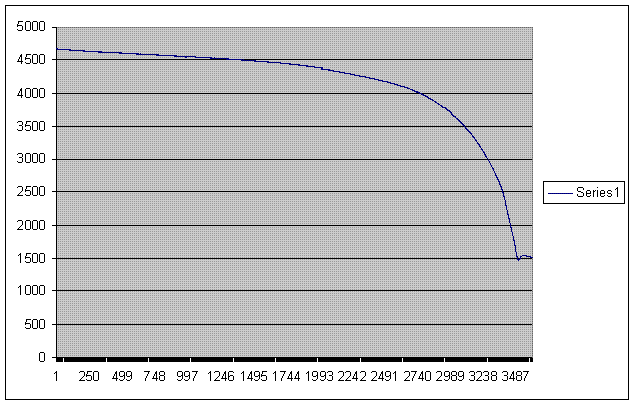
I've read up on batteries today and haven't came across anything like this... Maybe I DID have a bad connection... Anyway, here is a graph of what happened to the voltage on the battery RIGHT after I disconnected the charger(solar panel) when it was fully charged. Each x axis value is a 5 second interval.
-Phil
Another question, was the blub still illuminated in this region?
▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔
Paul Baker
Propeller Applications Engineer
Parallax, Inc.
With this setup, I am logging the battery voltage every 5 seconds from the propeller, onto an SD card. From there, I pull the data into Excel and graph it. Once the battery is charged, I will do the same in reverse; discharge the battery through a 10 ohm resistor and log the voltage. Ideally, I suppose I should be logging the current as well, but I know the battery charger voltage is fairly constant upstream of the resistor during charge, and I will know the discharge current since I know the resistance and the battery voltage.
Anyway, this is something very simple but I'm just amazed at how easy and sophisticated the things I can do with the propeller are.
I wonder if the glitch was from strong cells capable of retaining a decent charge.
Anyway, notice the behavior at the end. It goes down quickly, levels off, then goes down again... Like I said, this battery was used in some telecom equipment as backup power. I don't know if it was used extensively, but I only got about 20AH our of it.
-Phil
This discharge rate is refered to as C, so standard discharge is 1C, 1/2 discharge rate is 0.5C, 2 x discharge rate is 2C and so on. When discharge rate is < 1, the observed capacity of the cell is greater than what the battery is rated at. When the discharge rate > 1, the observed capacity of the cell is less than what the battery is rated at.
Another factor which can skew the observed capacity from the rated capacity is the temperature of the cell. Nearly all chemical reactions occur at a faster rate when the temperature is raised, this is also true for batteries (which are really just electrochemical devices, converting chemical energy into electrical energy). Lowering the temperature will reduce the effective capacity, and if you lower it far enough no chemical reaction will take place. This is why radioisotope batteries are the gold standard for batteries used in space especially for deep space, radioactivity isn't effected by temperature nearly as much as chemical processes are, and the radioactivity is self-heating which keeps a floor temperature for the unit even in the coldest parts of space.
Here's a Maxim ap note discussing the issues: http://www.maxim-ic.com/appnotes.cfm/appnote_number/121
▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔▔
Paul Baker
Propeller Applications Engineer
Parallax, Inc.
Post Edited (Paul Baker (Parallax)) : 6/26/2008 6:17:30 PM GMT
@Phil - I bet you're right. This isn't a new battery, so I bet some cells are in better shape than others. The knee occured at less than 10.5V, which is below the "discharged" voltage anyway. I assume that one cell finally went kapoot, while the others still had a little life. Anyway, I'm recharging the battery at a constant rate, and logging the voltage.