Used the wrong solder 10 years ago
 xanadu
Posts: 3,347
xanadu
Posts: 3,347
Plumbing solder. The circuit is two LM3915s, and it all still works if you hold it just right.
This board was soldered in 2003, picture taken today.
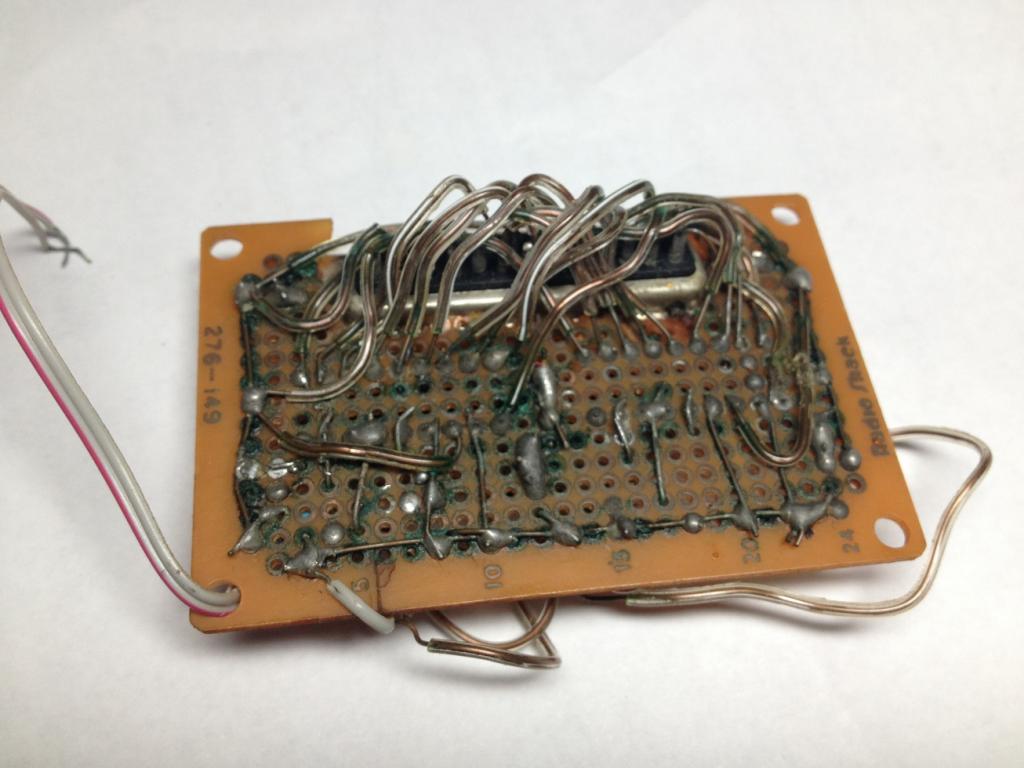
This board was soldered in 2003, picture taken today.



Comments
The acid flux corrodes heavily around electricity; the rosin flux is preferred. But these days, I think all the copper pipes are silver soldered to avoid lead... rather silly as tin alone would do nicely.
Not so. Pure tin, as a solder, has several problems, most of which are avoided with the addition of small quantities of alloying metals.
This should get the acid and grease off the board.
Duane J
Plumbing and automotive solders can contain an acid flux core, commonly Muriatic/Hydrochloric Acid. This reacts with Copper to form Copper Chloride, a light brown compound that absorbs moisture turning a blue-green color.
Ten years! I seldom remember the drive to work in the morning. :-)
@
Hydrochloric acid does not react with copper. It reacts with iron, zink, but not with copper. Actually only the protons react with iron or zink, but they are unable to oxidize copper, look for the reduction potential of H+ -> 1/2 H2 and Co(0) -> Co+ or Co++ and you will see that it lays at the "wrong" side of the table
You may got confused because the so-called "acid" for etching boards reacts of course with copper, but that is Fe3+
I am not sure what is meant by 'automotive solder'. If it is electrical connections it is one flux, if it is not electrical it is the other.
And, I've no idea if the acid core is hydrochloric acid or some other acid flux.
I've seen a few YouTube videos where people make their own etchant by mixing hydrochloric acid and hydrogen peroxide. It turns a brilliant green as it works on the board. So how does that work?
@Martin_H: Look what reaction hydrogen peroxyde in acidic media has
I've been using Oatey #5 Soldering Paste forever.
It's very difficult to clean off.
I have not found it's solvent.
When doing solder connections on my small electronics projects
I always use 60/40 Rosin Core solder after dipping it in my Oatey#5 paste.
When doing this, my joints do not fail and the solder actually sticks to whatever I'm soldering.
My projects are pretty short lived, but I do have some that are 30 years old and still working.
I maybe missed someone saying what soldering paste etc they are using.
Thanks
I think this fits into this discussion.
If you look at some of the older Tektronix pages and materials, they used to actually power wash their scopes when they came in for service. Then bake then test, etc....
Ack! You're right, I stand corrected. ;-)
According to my book on inorganic chemistry the coinage metals (copper/silver/gold) in general resist non-oxidizing acids (such as HCl),
but mixing in an oxidizer changes that.
For instance conc. nitric acid doesn't dissolve gold until you add some conc sulfuric acid (the archetypal oxidizing acid). Copper reacts with carbonic
acid (dissolved CO2) only when oxygen is present I believe (this is what turns copper roofing green). This is the same sort of reaction
that acid flux residue enables I suspect.
Thanks for the explanation.